
Palladium-Catalyzed Carbonylation of (Hetero)Aryl, Alkenyl and Allyl Halides by Means of N-Hydroxysuccinimidyl Formate as CO Surrogate | The Journal of Organic Chemistry

Scope and Mechanism of Allylic C−H Amination of Terminal Alkenes by the Palladium/PhI(OPiv)2 Catalyst System: Insights into the Effect of Naphthoquinone | Journal of the American Chemical Society

Palladium-Catalyzed Carbonylation of (Hetero)Aryl, Alkenyl and Allyl Halides by Means of N-Hydroxysuccinimidyl Formate as CO Surrogate | The Journal of Organic Chemistry

Palladium-catalyzed decarboxylative asymmetric allylic alkylation of enol carbonates. - Abstract - Europe PMC

Palladium‐Catalyzed Allyl Cross‐Coupling Reactions with In Situ Generated Organoindium Reagents - Lee - 2011 - Chemistry – An Asian Journal - Wiley Online Library
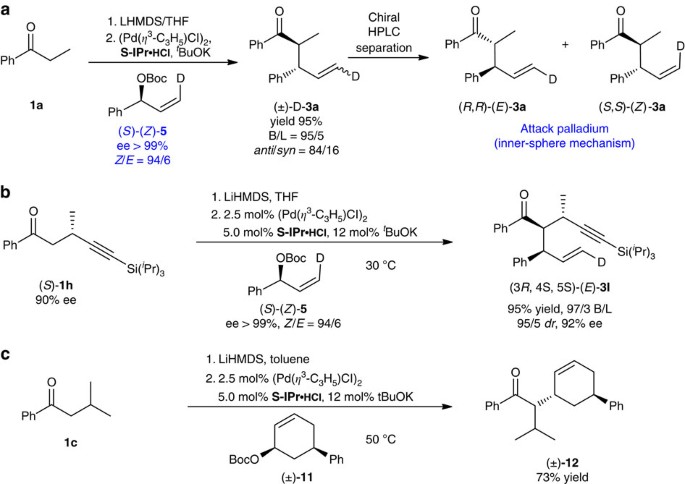
Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism | Nature Communications
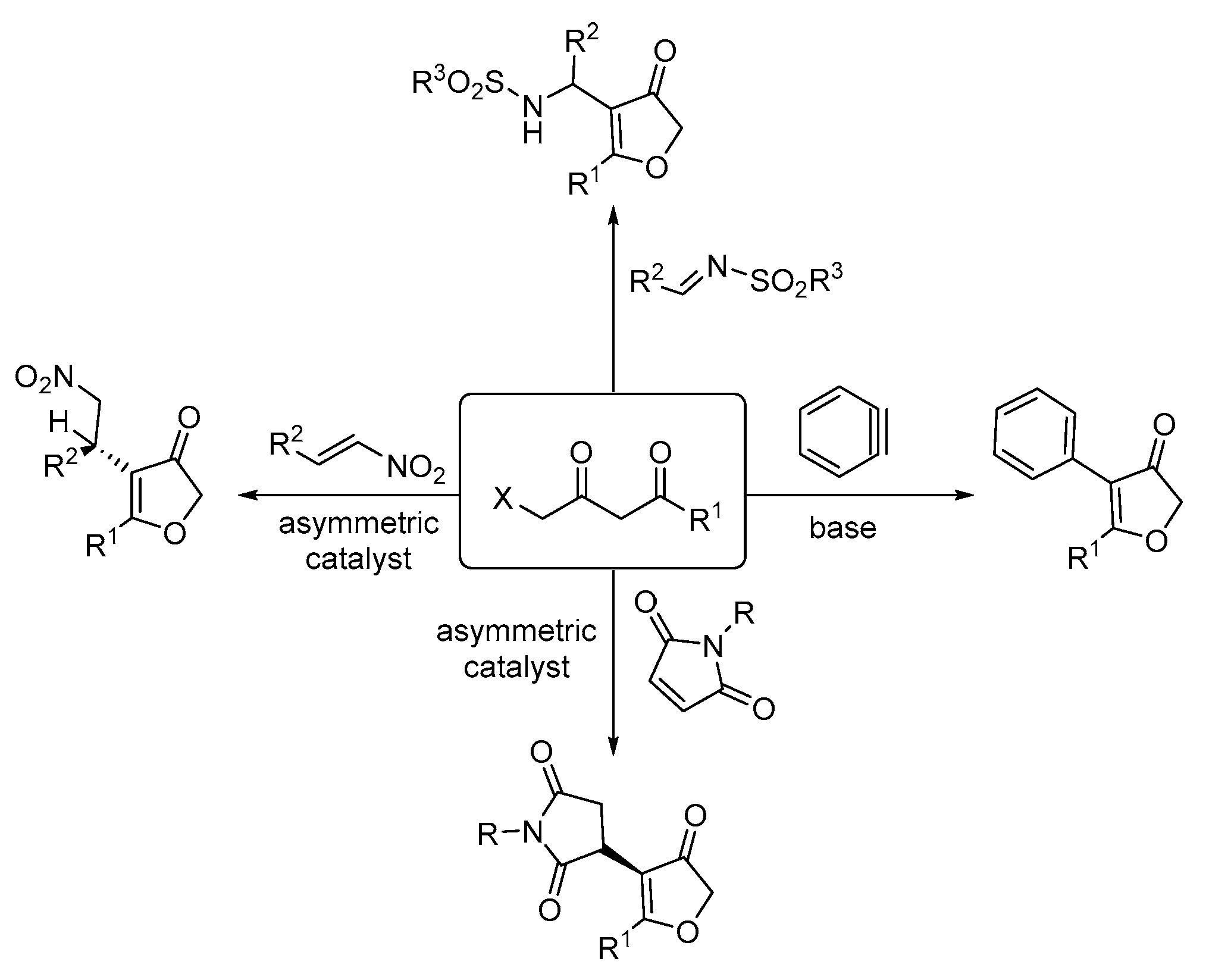
Organics | Free Full-Text | Palladium Catalyzed Ring-Opening of Diazabicylic Olefins with 4-Halo-1,3-Dicarbonyl Compounds: Accessing 3(2H)-Furanone-Appended Cyclopentenes
The Use of Palladium Catalysis for the Formation of Fused Aromatic Compounds and for the Diastereoselective Formate Reduction of
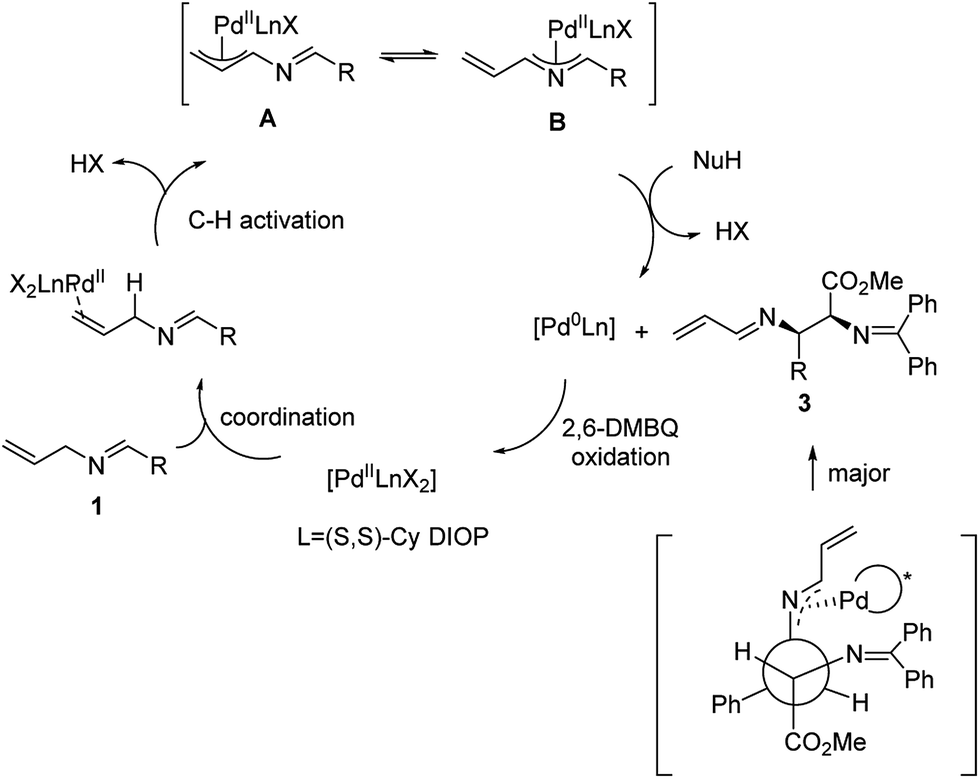
Pd-catalyzed asymmetric allylic alkylations via C–H activation of N -allyl imines with glycinates - Chemical Science (RSC Publishing) DOI:10.1039/C7SC02899G

Palladium‐Catalyzed Allyl Cross‐Coupling Reactions with In Situ Generated Organoindium Reagents - Lee - 2011 - Chemistry – An Asian Journal - Wiley Online Library

Scope and Mechanism of Allylic C−H Amination of Terminal Alkenes by the Palladium/PhI(OPiv)2 Catalyst System: Insights into the Effect of Naphthoquinone | Journal of the American Chemical Society
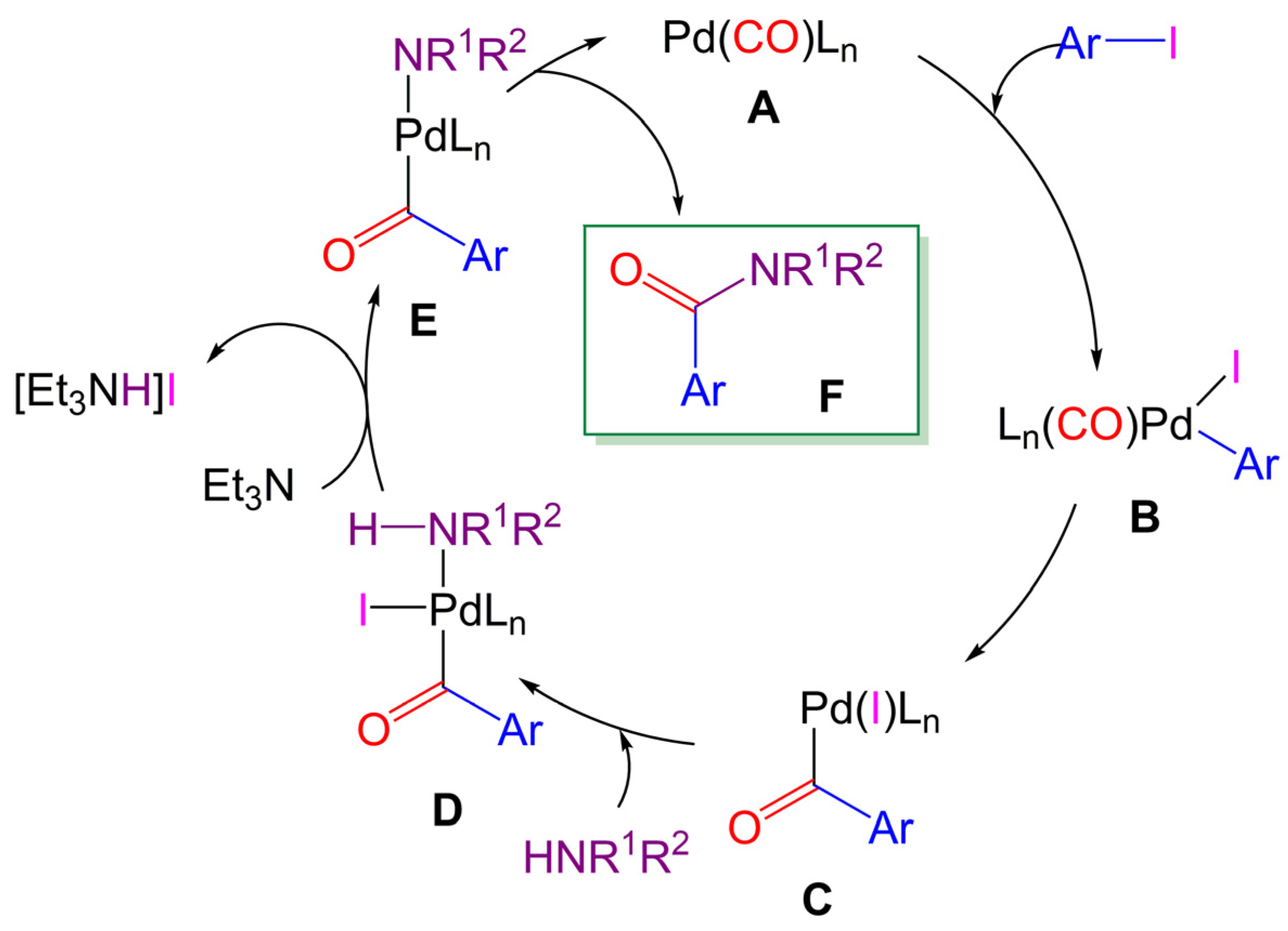
Molecules | Free Full-Text | Alkyl Levulinates and 2-Methyltetrahydrofuran: Possible Biomass-Based Solvents in Palladium-Catalyzed Aminocarbonylation

Palladium‐Catalyzed Allyl Cross‐Coupling Reactions with In Situ Generated Organoindium Reagents - Lee - 2011 - Chemistry – An Asian Journal - Wiley Online Library
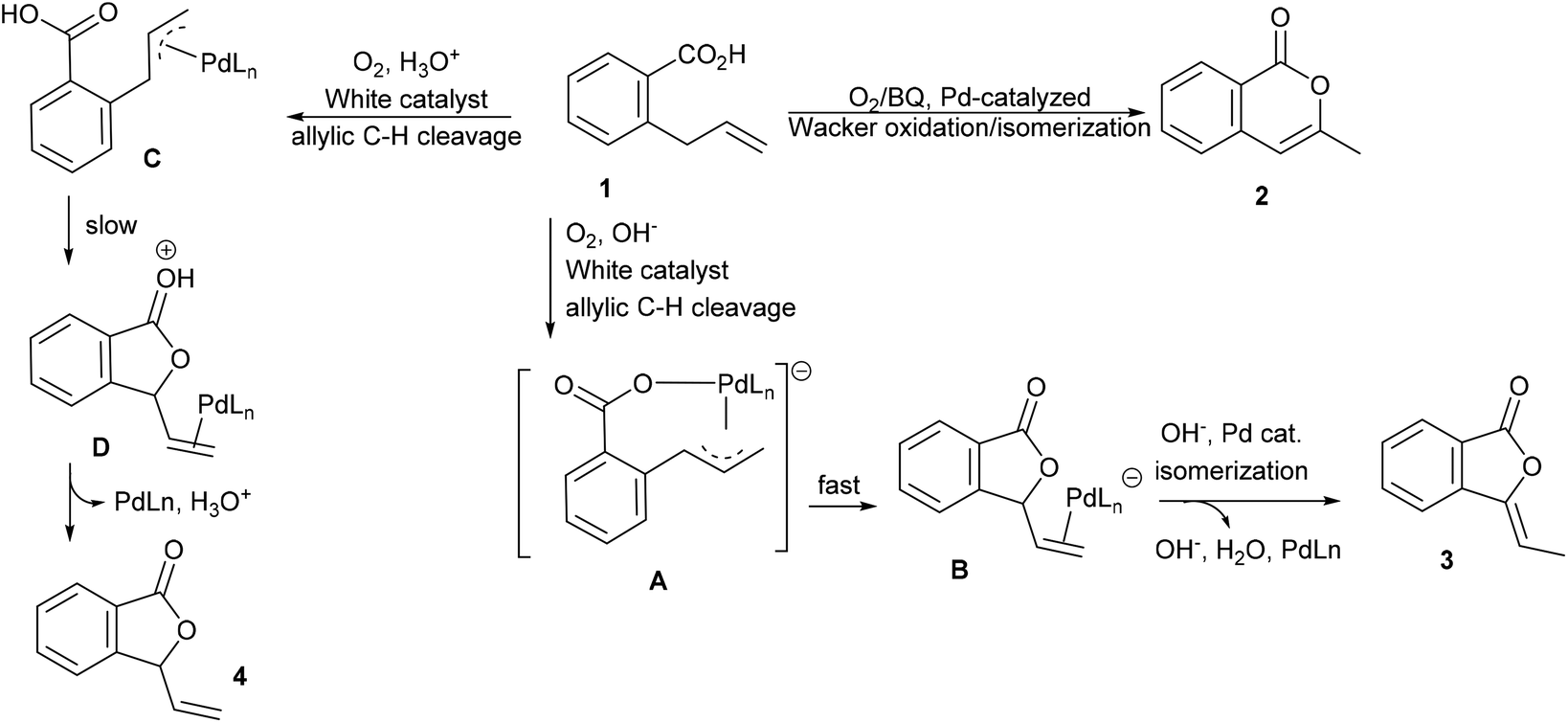
Acid–base-sensitive allylic oxidation of 2-allylbenzoic acids to form phthalides - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D0OB00303D

Scope and Mechanism of Allylic C−H Amination of Terminal Alkenes by the Palladium/PhI(OPiv)2 Catalyst System: Insights into the Effect of Naphthoquinone | Journal of the American Chemical Society

Sulfonamide- and hydrazine-based palladium catalysts: Stable and efficient catalysts for C–C coupling reactions in aqueous medium - ScienceDirect








